The electric charge or
simply charge is the subatomic
property of all matter. The electric charge is denoted by “Q” or “q” and is measured in coulomb.
Basically, the electric charge is the property by virtue of which a substance
shows electrical behavior.
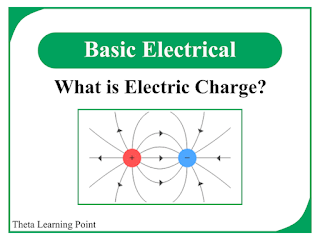
The important points about the electric charge are:
- In 1 coulomb of charge, the number of electrons (n) is
`\n=\frac{1}{1.6×10^{-19}}=6.24×10^{18}" "e^-`
- The charge is quantized, i.e. the charges that occur in nature are always the integral multiples of electronic charge. That means, there is no ½ C, 3/2 C, etc. existing in nature.
Charge, Q = ne ; Where, n = 0, 1, 2, ...
- The charge of a given system is always conserved, i.e. the law of conservation of charge states that the charge cannot be created or cannot be destroyed. Therefore, the algebraic sum of electric charges in a system does not change.
Properties of Electric Charge
The following are the two important properties of electric
charge:
- Like charges, i.e. positive and positive charges or negative and negative charges, repel each other.
- Unlike charges, i.e. positive and negative charges, attracts each other.
Numerical Example - How much electric charge is flowing in a circuit where `\1.37×10^{15}` electrons move past a given point.
Solution - Given data,
`\"Number of electrons",n=1.37×10^{15}`
`\∵"Charge",Q = n e`
`\∴Q=(1.37×10^{15})×(1.6×10^{-19})`
`\Q=2.192×10^{-4}" C"`

.png)




0 Comments